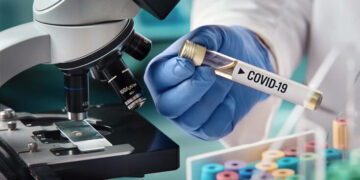
Το Ηνωμένο Βασίλειο ετοιμάζεται να γίνει η πρώτη χώρα στην Δύση που θα εγκρίνει το εμβόλιο του κορωνοϊού, με την ανεξάρτητη ρυθμιστική Αρχή να το εγκρίνει εντός ημερών, σύμφωνα με τους Financial Times.
Οι παραδόσεις του εμβολίου που αναπτύχθηκε από τις Pfizer/BioNTech θα ξεκινήσουν μέσα σε λίγες ώρες από την έγκριση, σύμφωνα με πηγές του Whitehall. Άτομα με γνώση της διαδικασίας δήλωσαν ότι τα πρώτα εμβόλια θα μπορούσαν να πραγματοποιηθούν από τις 7 Δεκεμβρίου.
Το Ηνωμένο Βασίλειο έχει παραγγείλει 40 εκατομμύρια δόσεις του συγκεκριμένου εμβολίου των δύο δόσεων, τα προκαταρκτικά δεδομένα του οποίου έδειξαν ότι είναι περισσότερο από 95% αποτελεσματικό στην πρόληψη του κορωνοϊού.
Τα εμβόλιο κανονικά πρέπει να πάρουν έγκριση από τον Ευρωπαϊκό Οργανισμό Φαρμάκων έως το τέλος της μετάβασης στο Brexit στις 31 Δεκεμβρίου. Ωστόσο, η Ρυθμιστική Υπηρεσία Φαρμάκων και Προϊόντων Υγείας του Ηνωμένου Βασιλείου έχει την εξουσία να εγκρίνει προσωρινά προϊόντα, σε περιπτώσεις επείγουσας ανάγκης του κοινού. Η ίδια διαδικασία θα μπορούσε να εφαρμοστεί στο εμβόλιο που αναπτύχθηκε από το Πανεπιστήμιο της Οξφόρδης σε συνεργασία με την AstraZeneca. Την Παρασκευή, η βρετανική κυβέρνηση έστειλε επιστολή στην ρυθμιστική Αρχή, ζητώντας της να επανεξετάσει το εμβόλιο AstraZeneca – Οξφόρδης.
Η Ντάουνινγκ Στριτ ανακοίνωσε σήμερα το πρωί πως ο Νατνίμ Ζαχάουι, υφυπουργός στο Υπουργείο Επιχειρήσεων, θα αποσπαστεί στο Υπουργείο Υγείας ως υπουργός για την επίβλεψη της διάθεσης των εμβολίων κορωνοϊού.
Αναμένεται έγκριση το εμβολίου της Pfizer και στις ΗΠΑ – Ίσως μέχρι τις 8-10 Δεκεμβρίου
Η BioNTech και η Pfizer νωρίτερα αυτό τον μήνα υπέβαλαν δεδομένα από την τρίτη φάση της κλινικής δοκιμής του εμβολίου, στην οποία συμμετείχαν περισσότερα από 43.000 άτομα, στην Αμερικανική Υπηρεσία Τροφίμων και Φαρμάκων. Μια έκτακτη έγκριση του εμβολίου στις ΗΠΑ Θα μπορούσε να έρθει το συντομότερο στις 8-10 Δεκεμβρίου, με αποστολές σε όλη την χώρα να ξεκινούν εντός 24 ωρών από την ανακοίνωση σύμφωνα με αμερικανικά δημοσιεύματα.
Πηγή: iefimerida.gr